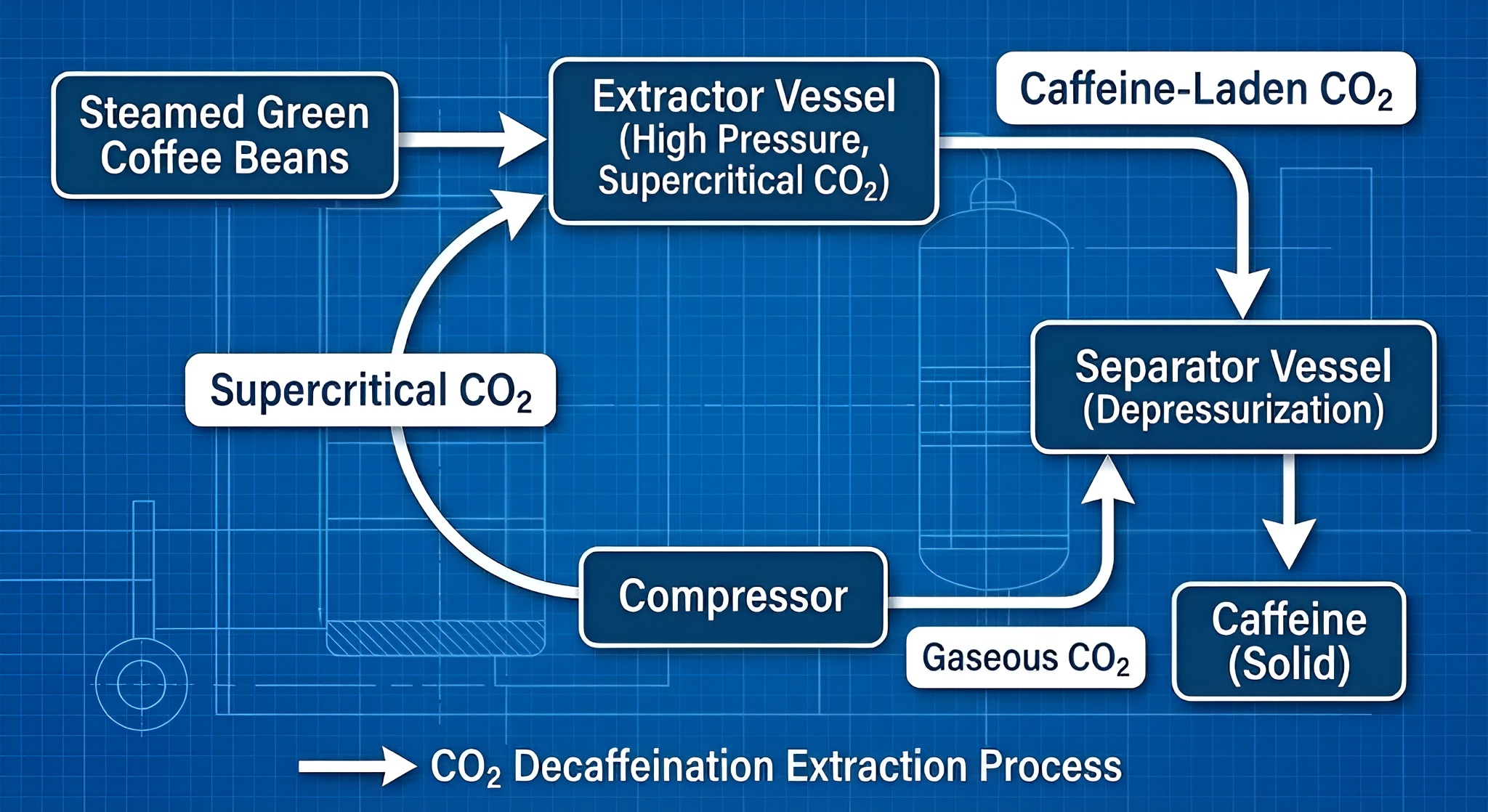
1. The CO2 Method: The Premium Choice
This is the most modern and expensive method. Tea leaves are moistened and placed in a high-pressure stainless steel chamber. Carbon Dioxide (CO2) is pumped in until it reaches a "supercritical" state—it becomes a liquid-gas hybrid.
This supercritical CO2 acts as a magnet for caffeine molecules, pulling them out of the leaf while leaving the larger flavor molecules (tannins, polyphenols) intact. When the pressure is released, the CO2 evaporates instantly, leaving zero chemical residue.
Expert Tip: Why does it cost more?
The CO2 method requires massive, high-pressure machinery that is expensive to operate. This is why a box of CO2 decaf tea (like Clipper or Arbor) costs more than standard supermarket decaf.
2. Methylene Chloride vs. Ethyl Acetate
These are the two main "solvent" methods. The tea is soaked in the chemical, which bonds to the caffeine, and then the chemical is evaporated off.
- Methylene Chloride (MC): It bonds very specifically to caffeine, so it preserves the tea's flavor better than water processing. However, consumers worry about trace residues, despite strict regulations.
- Ethyl Acetate (EA): Found naturally in bananas and apples, so brands label it "Natural." However, it is harder to remove than MC and often leaves a persistent chemical/fruity taste that alters the tea's profile.
3. Why Not "Swiss Water"?
The Swiss Water Process is famous for coffee. It involves soaking beans in hot water to dissolve caffeine. However, hot water also dissolves tea flavor (that's how you brew tea!).
With coffee beans, you can soak them and then dry them out. With tea leaves, soaking them in hot water essentially "brews" the tea before you ever buy it. The resulting leaf is fragile and tasteless. That is why water decaffeination is rarely used for high-quality tea.
| Method | Chemicals Used? | Flavor Retention | Cost |
|---|---|---|---|
| CO2 (Supercritical) | No (CO2 Gas) | High (90%+) | $$$ |
| Methylene Chloride | Yes | Medium-High | $ |
| Ethyl Acetate | Yes ("Natural") | Low (Chemical taste) | $ |
| Water Process | No | Very Low | $$ |
Ready to upgrade your evening brew?
We've done the research to find brands that exclusively use the CO2 method, ensuring you get a clean cup without the chemical aftertaste. The 5 Best CO2 Decaf Teas of 2025 →
Comments